MONITORING THERAPY
Early Determination of Response to Therapy
A baseline blood sample is taken before therapy. A second blood sample is taken about 30 days after the therapy. The response to therapy is determined by comparing the results of the two samples:
Not working: Any CTCs detected in the second sample
Not working: CAML size or number increased in the second sample
Some benefit from therapy: CAML size or number decreased in the second sample
Ideal case: no CAML or CTC in the second sample
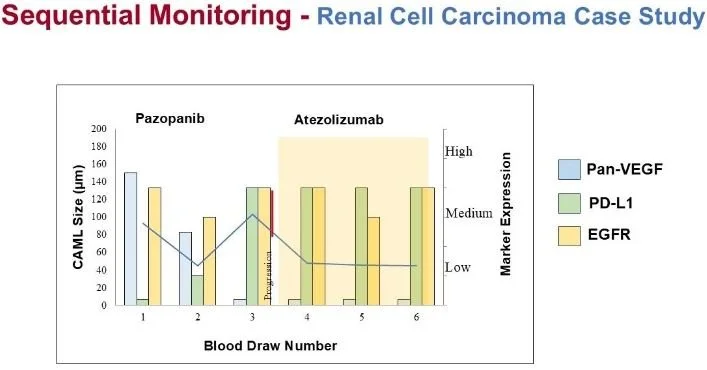
Sequential Monitoring of Tumor Marker Status During Therapy
Sequential monitoring of therapy is recommended, because tumors change. This is especially important when the drug is targeting a specific marker.
Case Study:
Patient was initially on Pazopanib, which targets pan-VEGF. The original expression of pan-VEGF was high, but it decreased over time. Patient’s cancer progressed as indicated by an increase in CAML size.
At the same time, the expression of PD-L1 became high. The therapy was switched to Atezolizumab, an immunotherapy drug. The patient’s CAML size decreased following the therapy.
This patient sample was processed to analyze three companion diagnostic markers: Pan-VEGF, PD-L1, and EGFR.
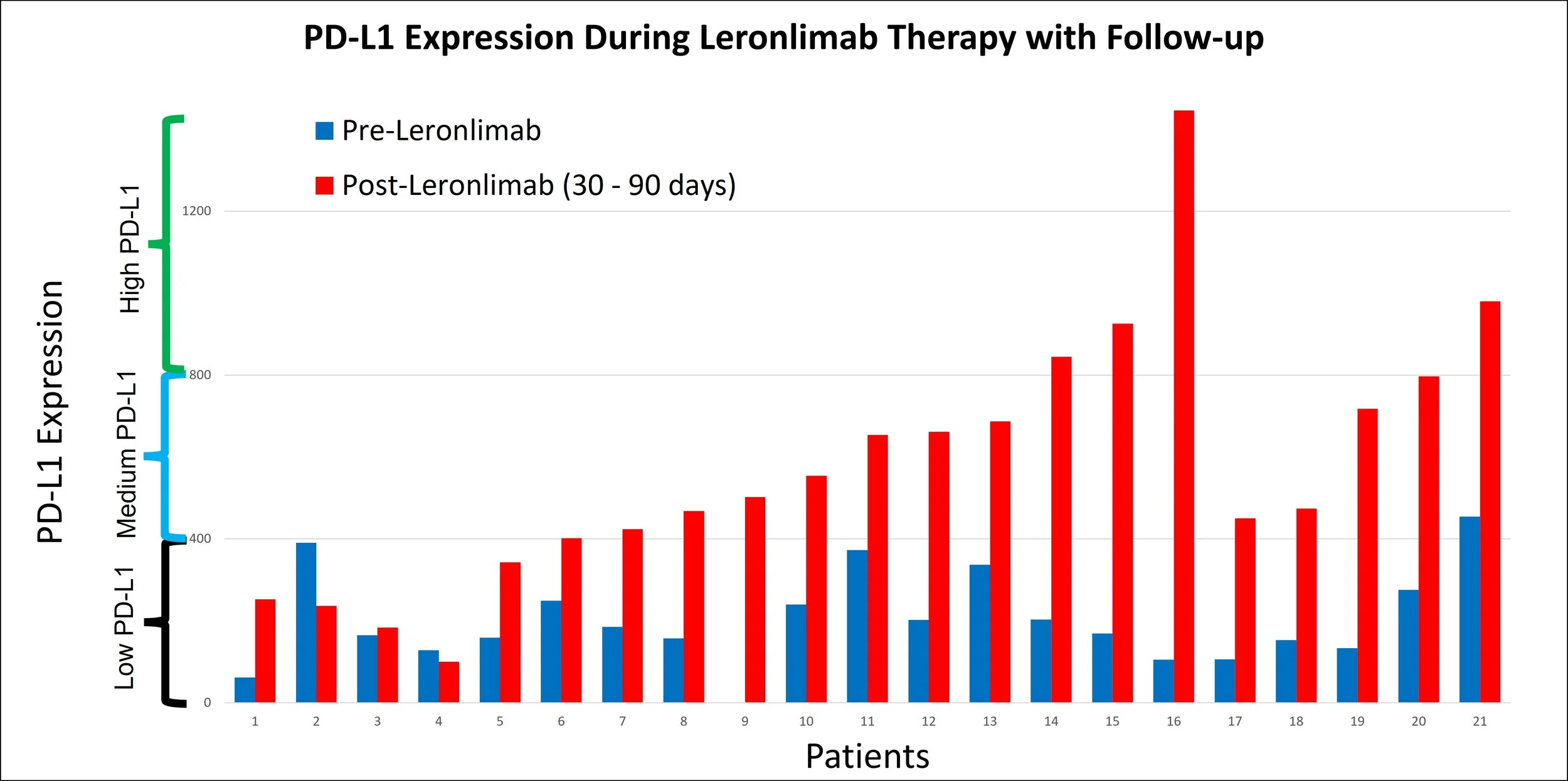
Monitoring Change of PD-L1 Expression
Immunotherapies work the best when the PD-L1 expression on the tumor is high. Our clinical research shows that PD-L1 expression can change under a variety of situations.
Some examples that we have published on are related to changes during (i) chemoradiation therapy, (ii) Briacell’s cancer vaccine therapy, (iii) renal cell carcinoma case study above, and (iv) CytoDyn’s Leronlimab breast cancer trial. The data shown is from the CytoDyn clinical trial. The majority of PD-L1 expression after therapy is much higher than before therapy.